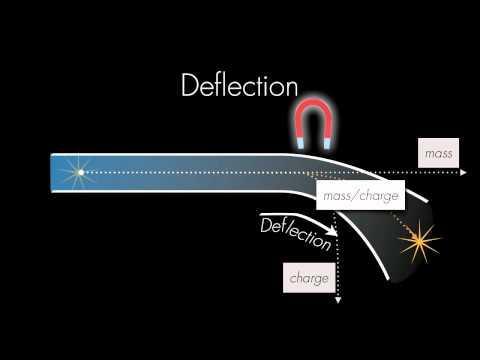
In 1919, Francis Aston invented the mass spectrometer. It stripped electrons from an atom and used an electric field to injected the positively charged, ionized atoms through two slits into a strong magnetic field. The magnetic field sorted elements and isotopes of elements according to their mass. This eventually allowing Aston to identify 212 of the 281 naturally occurring isotopes. Each element contained a number of protons that described its chemistry but many elements had isotopes containing a variable number of neutrons.
This explained why atomic weights were not all exactly multiples of one. However, it raised other questions. The atomic weights of all isotopes were very nearly whole numbers with the odd exception of hydrogen (the most common isotope having one proton) at 1.008. But why was the atomic weight of helium (with 2 proton and 2 neutrons) exactly 4.000 and not 4.032?
Aston reasoned that the missing mass was caused by the energy required to bind the protons and neutrons together. Without the neutrons the positively charged protons would repel each other and the nucleus could not exist. If four hydrogen atoms could be combined into one helium atom, this would release an enormous amount of energy (as produced in the Sun) according to Einstein's famous formula (Energy = mass times the velocity of light squared).
Aston noted that elements in the middle of the periodic table were more stable than those at both ends, implying that the heaviest atoms would release energy if they could be broken down.
In 1930, in Germany, Walter Bothe and Herbert Becker bombarded light elements from lithium to oxygen with alpha radiation from a polonium source to measure the gamma radiation. They were astonished when beryllium exhibited ten times the radiation intensity of other elements, without emitting protons. The energy released was greater than that of the bombarding alpha radiation!
In Paris, Marie Curies' daughter, Iréne, and Frédérick Joliot found that 222-radon gas spontaneously transmuted itself into three radio-active isotopes, 210-lead, 210-bismuth and 210-polonium.For decades doctors had been using small glass ampoules, 'seeds' of radon gas to treat cancer and many of these had been returned to Paris when they had decayed. One of the many decay products of radon was 210-polonium, with a half life of 138.376 days it was the most intense source of radiation known at that time (a milligram of 210-polonium emits as many alpha particles per second as 5 grams of 226-Radium) and the Joliot-Curies had extracted the largest amount in the world.
After Rutherford's discoveries of 1932, scientist quickly accepted the idea that atomic nucleus was composed of protons and neutrons, although the neutron's function was not initially understood. However, within months, Werner Karl Heisenberg and Dmitri Ivanenko had proposed proton–neutron models for the nucleus; Heisenberg using quantum mechanics.
Between 1925 and 1927, Werner Heisenberg, a German theoretical physicist, working with Max Born and Pascual Jordan, developed the matrix formulation of quantum mechanics and the Uncertainty Principle. Little understood at the time, it proved essential to the understanding of the properties of very small particles.
It was the first theory of nuclear exchange forces that bind the nucleons (protons and neutrons) and was a landmark to understanding the nucleus as a quantum mechanical system. Quantum mechanics developed gradually in the mid-1920's when many scientist including Erwin Schrödinger, Niels Bohr, Max Born, Paul Dirac and Werner Heisenberg, could not explain observations using traditional (classical) physics. Max Planck's solution, in 1900, to the black-body radiation problem and Albert Einstein's 1905 paper on the correspondence between energy and frequency (which explained the photoelectric effect) were early attempts to understand phenomena at the scale of the nucleus. How electro-magnetic radiation can behave both as a wave and as particles (quanta) has still not been explained.
Quantum mechanics differs from classical physics in that energy, momentum, angular momentum, and other quantities of a bound system are restricted to discrete values (quantization). Also, objects have characteristics of both particles and waves (wave-particle duality), and there are limits to how accurately the value of a physical quantity can be predicted prior to its measurement, given a complete set of initial conditions (the uncertainty principle).
Heisenberg considered protons and neutrons to be different quantum states of the same particle; nucleons distinguished by the value of their nuclear isospin quantum numbers. But, while Heisenberg's theories broke new ground and hugely influenced later research work, his ideas were so radical that when he submitted a paper to the journal Nature, it was rejected as being too speculative.
Heisenberg published his work in 1925 in a breakthrough paper and in subsequent papers with Max Born and Pascual Jordan, during the same year. He described the uncertainty principle in 1927 and was awarded the 1932 Nobel Prize in Physics.
In early 1929, Heisenberg and Wolfgang Pauli submitted the first of two papers laying the foundation for relativistic quantum field theory. In the spring of 1929, Heisenberg lectured on quantum mechanics at the University of Chicago.
In 1928, the British mathematical physicist Paul Dirac had derived his relativistic wave equation of quantum mechanics, which implied the existence of positive electrons, later to be named positrons.
In 1932, from a cloud chamber photograph of cosmic rays, the American physicist Carl David Anderson identified a track as having been made by a positron. In mid-1933, Heisenberg presented his theory of the positron. His thinking on Dirac's theory and further development of the quantum theory were set forth in two papers in 1934, and 1936. In these papers Heisenberg was the first to reinterpret the Dirac equation as a (quantum) field equation accurately describing electrons. Heisenberg put the matter on the same footing as electromagnetism; describing relativistic quantum field equations which allowed the possibility of particle creation and destruction. (Hermann Weyl had already suggested this idea in a 1929 letter to Albert Einstein).
https://www.youtube.com/watch?v=7kb1VT0J3DE&t=11s Quantum 8

YOU ARE READING
Nuclear
Non-FictionH.Becquerel found uranium emitted radiation and, in1898, M.Curie extracted minute traces of radium from ore. J.Maxwell predicted electro-magnetic radiation in 1855 and Einstein formulated special relativity; mass could be converted into energy. In...